Which Is the Most Basic Nitrogen in Each Compound.
DMAP 4-NN-dimethylaminopyridine Transcribed Image Text. Name each ionic compound.
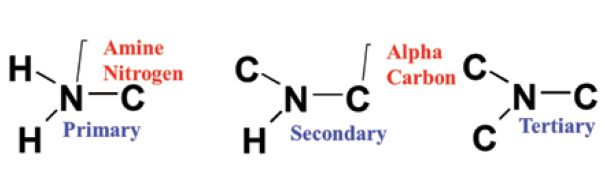
Organic Nitrogen Compounds Ii Primary Amines
Thoroughly explain your choice to merit full points.

. 135-Triazine is the strongest base. Order the anionic compounds from the most basic to least basic. I thought that the bond with more s-character increased in acidity but the answer is supposed to be 7.
Factors such as bulkiness around the nitrogen atom can also be considered while deciding basic strength. Is the same true for the analogue of nicotine that OneClass. Write chemical formulas for compounds containing each of the following.
See the answer See the answer See the answer done loading. Now the confusion is between compounds a and b. For compounds below identify the most basic nitrogen atoms.
For compounds below identify the most basic nitrogen atoms. 2021 Which is the most basic nitrogen in each compound. HintThe basic strength of a compound depends upon its ability to accept a proton from another moleculeNow one can apply this concept in the above structures and decide which structure can have more basic strength.
Therefore b is more basic because it has the weaker conjugate. 1 base 2 acid 3 acid. Normally you would always say that the S P three hybridized nitrogen is more basic.
What are the basic units-single atoms molecules or formula units-that compose each of the following substances. Oxygen as the more electronegative element holds more tightly to its lone pair than the nitrogenThe nitrogen lone pair therefore is more likely to break away and form a new bond to a proton it is in other words more basic. I think most of your examples can be reasonably thought out with these two key points.
Based on this c is the most basic and d the least. Why Is Nitrogen More Basic Than Oxygen. The most basic nitrogen in the following compound is A I B II C III D IV.
Thoroughly explain your choice to merit full points. Which is the most basic nitrogen in each compound. Organic compounds containing a carbonyl group bonded to a nitrogen atom are amides and the carbon-to-nitrogen bond is an amide linkage or a peptide linkage.
Imidazole forms part of the structure of the amino acid histidine and. The lone pair on nitrogen b is the most basic. Want to see the full answer.
Identify the most basic nitrogen atom on nicotine compound A and explain why. The conjugate acid of a is H 3 O which has a pKa of -2 and the conjugate acid of b is NH 4 which has a pKa of 9. Show transcribed image text.
Which is the most basic nitrogen in each compound. A b c Students also viewed these Organic Chemistry questions. The most basic nitrogen in the following compound is - Sarthaks eConnect Largest Online Education Community.
Chemical Reactions of Amines. Most amides are colorless and odorless and the lighter ones are soluble in water. And we want to circle which nitrogen atom is going to be more basic.
Identify the most basic nitrogen atom on nicotine compound A and explain why. View solution Which compound does not give positive test in Lassaignes test for nitrogen. With the exception of nitric acid these compounds are widely used as fertilizer.
If you cant remember the properties of common basic groups it is also possible to. Solve Study Textbooks Guides. Which of the following compounds has the most basic nitrogen.
Identify each compound as an acid or a base. Draw resonance structures where appropriate. Up to 256 cash back Get the detailed answer.
Check out a sample QA here. Which of the following compound is the most basic. View solution In each of the following pair of compounds which is more basic in aqueous solution.
Most basic 1 second basic 2. Therefore a is less basic and b is more basic. Referring to the molecule above I know that pyridine C 6 H 5 N is a stronger base than water which I consider a type of alcohol so N is the most basic atom in this molecule.
2021 Which is the most basic nitrogen in each compound. The more electronegative an atom the better it is able to bear a negative charge. For the first molecule for example the double bonded nitrogen is less basic due to increasing s character closer to nucleus less likely to bind to H.
To decide the strength of a and b notice that in b there is just one nitrogen pulling electron density away inductive effect while in. Ammonia is converted to other nitrogen compounds the most important of which are urea NH2CONH2 nitric acid HNO3 ammonium nitrate NH4NO3 and ammonium sulfate NH42SO4. 135-Triazine 1 The lone pairs on N are in sp2 orbitals in the plane of the ring.
They cannot interact with the aromatic π system so they are all available for bonding. I understand that the lone pairs of the Nitrogens on the left ring are tied up in resonance and on the right are tied up in aromaticity but I still dont see how you can factor all that in to answer the. One nitrogen atom for every three chlorine atoms.
2 pts 025 pts each el NH NH Question. In each of these compounds the metal forms only one type of ion. You must be signed in to discuss.
Which is the most basic nitrogen in each compound. What type of orbital overlap is responsible for the sigma bond between carbon and nitrogen in the molecule below. AFIGURE CANNOT COPY bFIGURE CANNOT COPY cFIGURE CANNOT COPY Answer.
The reason for this is that the S P three orbital has further electron density right has more p characteristics that loan Paris further when easier to take on. So in the first case we have both an s P three and an S p to hybridize nitrogen. This problem has been solved.
Which nitrogen atom in each compound is more basic. Want to see the full answer. The question asks which nitrogen is most basic out of 2 1 7 or 9.

A Nice Summary Of Heterocycles Organic Chemistry Organic Chemistry Organic Chemistry Study Chemistry Posters

Nitrogen Is A Chemical Element With The Symbol N And Atomic Number 7 Dinitrogen N2 Forms About 78 Chemistry Education Chemistry Experiments Chemistry Jokes

Nitrogen Compound An Overview Sciencedirect Topics

Amines Y Học Nong Nghiệp Sinh Học Phan Tử
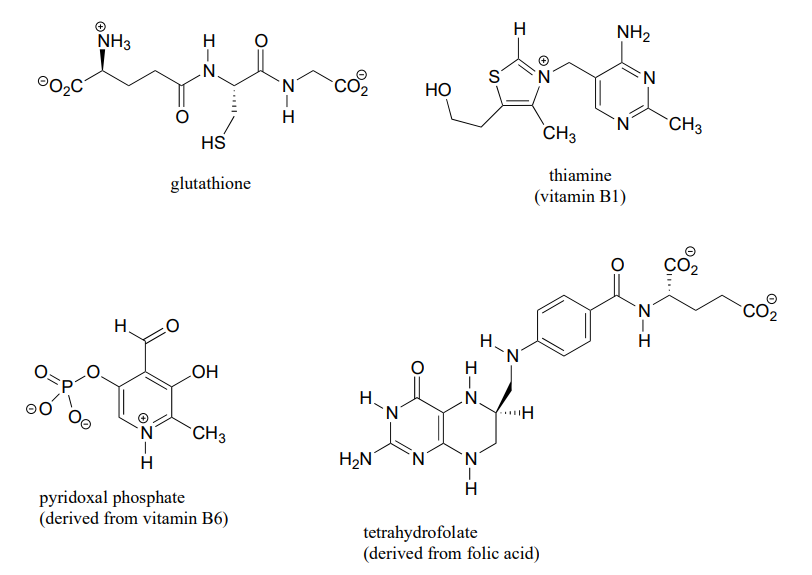
7 6 Acid Base Properties Of Nitrogen Containing Functional Groups Chemistry Libretexts

Pyridine Pyridine Chemical Formula Benzene
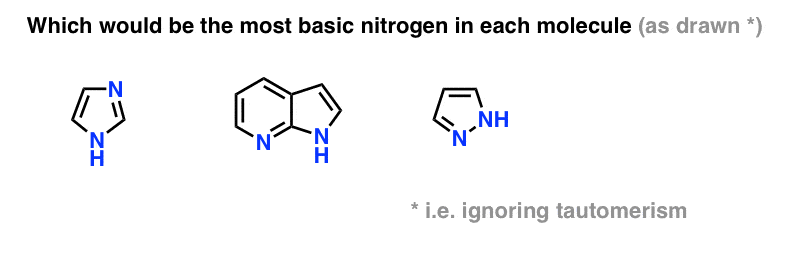
5 Key Basicity Trends Of Amines Master Organic Chemistry

Worksheets Periodic Table Carbon Periodic Table Of The Elements Science Worksheets Homeschool Science

5 Key Basicity Trends Of Amines Master Organic Chemistry
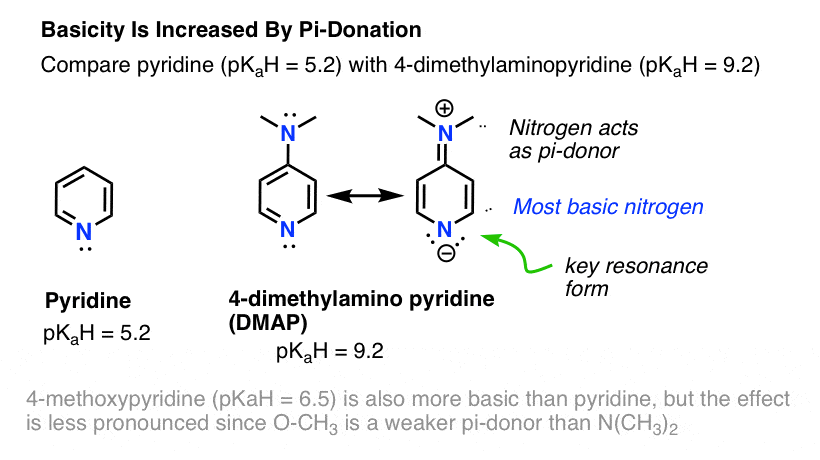
5 Key Basicity Trends Of Amines Master Organic Chemistry

Nitrogen An Overview Sciencedirect Topics

Periodic Table Nitrogen Worksheet Education Com Nitrogen Physical Science Science Worksheets
How To Identify The Most Basic Atom
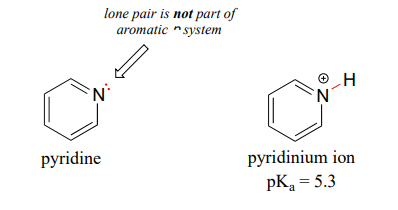
7 6 Acid Base Properties Of Nitrogen Containing Functional Groups Chemistry Libretexts

Nitrogen Compound An Overview Sciencedirect Topics

Lesson Marshmallow Molecules Teaching Chemistry Matter Worksheets Basic Anatomy And Physiology
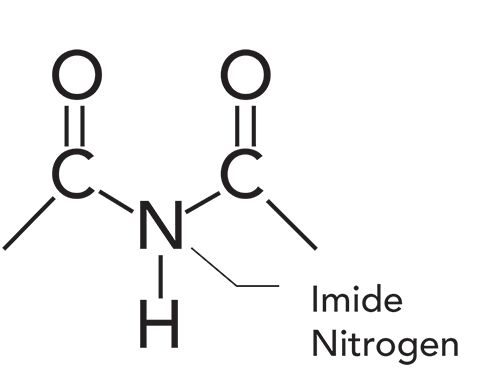
Organic Nitrogen Compounds Viii Imides


Comments
Post a Comment